Analysis of Residual Solvents in Pharmaceuticals by Water-Soluble Samples Using N2 Carrier
The Japanese Pharmacopoeia 17th Edition (JP17) and the United States Pharmacopeia (USP) General Chapters <467> Residual Solvents provide test methods for residual solvents in pharmaceuticals mainly using headspace gas chromatography (GC). Residual solvents in pharmaceuticals are classified from Class 1 to 3 based on their potential human health risk. Since these compounds are strictly controlled, highly sensitive analysis is required. Helium (He) is generally used as the carrier gas, but as He supply shortages have become an issue recently, analysis using an alternative carrier gas such as N2 has also been in demand lately. This article introduces a JP17 Supplement II- compliant analysis of water-soluble samples for Class 1 and 2 residual solvents using N2 as the carrier gas.
Analysis Conditions
Class 1 and 2 standard solutions were prepared and measured in accordance with Procedure A and B of JP17 Supplement II, using Shimadzu HS-20 headspace gas sampler connected to Nexis™ GC-2030 gas chromatograph. The two procedures differ in the type of column, the column temperature and the split ratio. Table 1 lists the GC and HS20 analysis conditions used in this experiment.
Table 1 Water-Soluble Sample Analysis Conditions
GC analysis conditions (Procedure A and B)
| Model | Nexis GC-2030 |
| Detector | FID-2030 flame ionization detector |
| Column |
A) SH-Rxi™-624 Sil MS (0.32 mm I.D.×30 m, d.f.= 1.8 μm)
B) SH-Stabiliwax (0.32 mm I.D.×30 m,
d.f.= 0.25 μm) |
| Column temperature | A) 40 ℃ (20 min) ‒ 10℃/min ‒ 240℃ (20 min) Total 60 min B) 50 ℃ (20 min) ‒ 6 ℃/min ‒ 165 ℃ (20 min) Total 59.17 min |
| Injection Mode | A) Split 1:5 B) Split 1:10 |
| Carrier Gas Controller | : Linear velocity (N2) |
| Linear Velocity | 35 cm/sec |
| Detector Temperature | 250 ℃ |
| FID H2 Flow Rate | 32 mL/min |
| FID Make up Flow Rate | 24 mL/min (N2) |
| FID Air Flow Rate | 200 mL/min |
| Injection Volume | 1 mL |
HS-20 analysis conditions (same for Procedure A and B)
| Oven Temperature | 80 ℃ |
| Sample Line Temperature | 110 ℃ |
| Transfer Line Temperature | 120 ℃ |
| Vial Stirring | Off |
| Vial Volume | 20 mL |
| Vial Heat-retention Time | 60 min |
| Vial Pressurization Time | 1 min |
| Vial Pressure | 75 kPa |
| Loading Time | 0.5 min |
| Needle Flush Time | 5 min |
Analysis of Class 1 Standard Solution (Water-Soluble Sample)
The analysis results for Procedure A and B are shown in Fig. 1 and 2 respectively. Table 2 and 3 summarize the S/N ratios and the repeatability of Procedure A and B respectively. The results obtained with Procedure A satisfied the requirements of JP17 Supplement II which states that “the S/N ratio for 1,1,1-trichloroethane in the Class 1 standard solution is not less than 5 and the relative standard deviation of each peak area is not more than 15 %.” Satisfactory results were also obtained with Procedure B, as “the S/N ratio for benzene in the Class 1 standard solution is not less than 5 and the relative standard deviation of each peak area is not more than 15 %.”
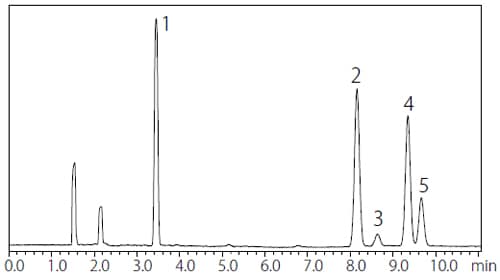
Fig. 1 Chromatogram of Class 1 Standard Solution by Procedure A (Water-Soluble Sample)
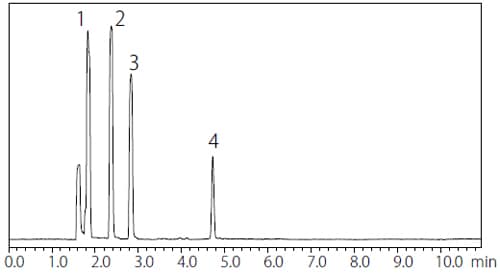
Fig. 2 Chromatogram of Class 1 Standard Solution by Procedure B (Water-Soluble Sample)
Table 2 S/N Ratio and Repeatability of Class 1 Standard
Solution (Procedure A)
| Peak No. | Compound | S/N ratio*1 | %RSD (n=6)**1 |
| 1 | 1,1-Dichloroethane | 263 | 1.81 |
| 2 | 1,1,1-Trichloroethane | 242 | 1.74 |
| 3 | Carbon tetrachloride | 18 | 2.72 |
| 4 | Benzene | 204 | 2.08 |
| 5 | 1,2-Dichloroethane | 69 | 1.56 |
Table 3 S/N Ratio and Repeatability of Class 1 Standard
Solution (Procedure B)
| Peak No. | Compound | S/N ratio*1*1 | %RSD (n=6)**1 |
| 1 | 1,1-Dichloroethane | 174 | 1.77 |
| 2 | 1,1,1-Trichloroethane | 181 | 2.18 |
| 3 | +Carbon tetrachloride | ||
| 4 | Benzene | 142 | 1.12 |
| 5 | 1,2-Dichloroethane | 105 | 1.02 |
In Table 2 and 3, the items specified in JP17 Supplement II are shown in red. *1 The S/N ratios and relative standard deviation (%RSD) are reference values and not intended to be guaranteed values.
Analysis of Class 2 Standard Solution (Water-Soluble Sample)
Fig. 3 and 4 below show the analysis results for Procedure A and B (Black: Class 2 mixture A standard solution (Class 2A), Pink: Class 2 mixture B standard solution (Class 2B), Blue: MIBK).
For system suitability, JP17 Supplement II specifies that “the resolution between acetonitrile and methylene chloride in the Class 2 mixture A standard solution is not less than 1.0” when using Procedure A, and “the resolution between acetonitrile and cis-1,2- dichloroethene in the Class 2 mixture A standard solution is not less than 1.0” when using Procedure B. Satisfactory results were obtained with both procedures.
* The resolutions shown in the Fig. 3 and 4 are reference values and not guaranteed.
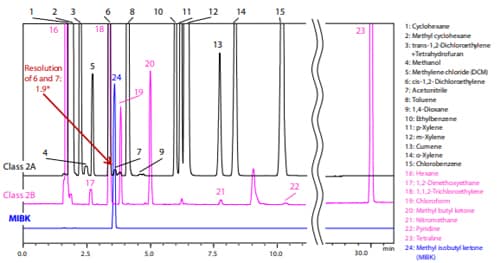
Fig. 3 Chromatogram of Class 2 Standard Solution by Procedure A
(Water-Soluble Sample)
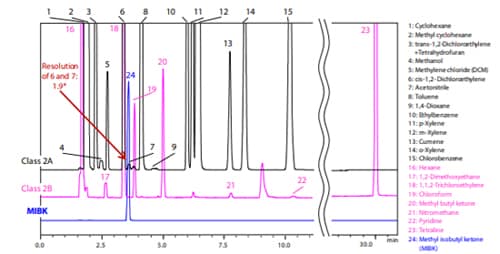
Fig. 4 Chromatogram of Class 2 Standard Solution by Procedure B
(Water-Soluble Sample)
In the analysis of residual solvents in water-soluble pharmaceuticals using the headspace GC method, satisfactory results comparable to those with He carrier gas were obtained with N2 as the carrier gas.



