Simultaneous Analysis of 97 Primary Metabolites By PFPP: Pentafluorophenylpropyl Column
Energy production is essential for every living organism. Energy production at the cellular level is carried out by various metabolic processes, including the glycolytic system and the TCA cycle. In order to investigate the metabolism of living systems, it is important to quantify the amount of each metabolite. However, many primary metabolites are hydrophilic and difficult to analyze by reversedphase chromatography. Ion pair chromatography is sometimes used for the analysis of such hydrophilic compounds but it is not preferable for LCMS analysis because ion pair reagents cause high background signals and sensitivity deterioration.
A new method using a Pentafluorophenylpropyl (PFPP) column has been developed to overcome these limitations. This data sheet presents the simultaneous analysis of 97 primary metabolites, such as amino acids, organic acids, nucleotides, nucleosides and co-enzymes, using a PFPP column, which uses particles functionalized with a pentafluorophenylpropyl group. It allows not only hydrophobic interaction but also retention of hydrophilic compounds, which is essential for successful analysis of primary metabolites.
Samples were tissue extracts. HPLC and MS experimental conditions include the LC/MS/MS method package for primary metabolites Version 2 and all measurements were done by Shimadzu LCMS-8040 Triple Quadrupole Ultra Fast Mass Spectrometer (UFMS), which features a 5 msec dwell time and 15 msec polarity switching rate.
■ Compound List (Color legend; TCA: ■ Methylation and Transsulfuration: ■ Urea: ■)
After excising liver and heart tissues from mice followed by immediate freezing in liquid nitrogen, each frozen sample was weighed and homogenized in methanol containing internal standards. After homogenization, methanol-chloroform extraction was carried out.
After collection of hydrophilic metabolites, extracts were concentrated. After preparation, 3 uL of diluted samples were injected on the LCMS-8040. Figure 1 illustrates overlaid MRM chromatograms of metabolites from liver and heart tissue extracts. In each extract, over 80 metabolites were detected. Major peaks and specific metabolites have been highlighted for each tissue.
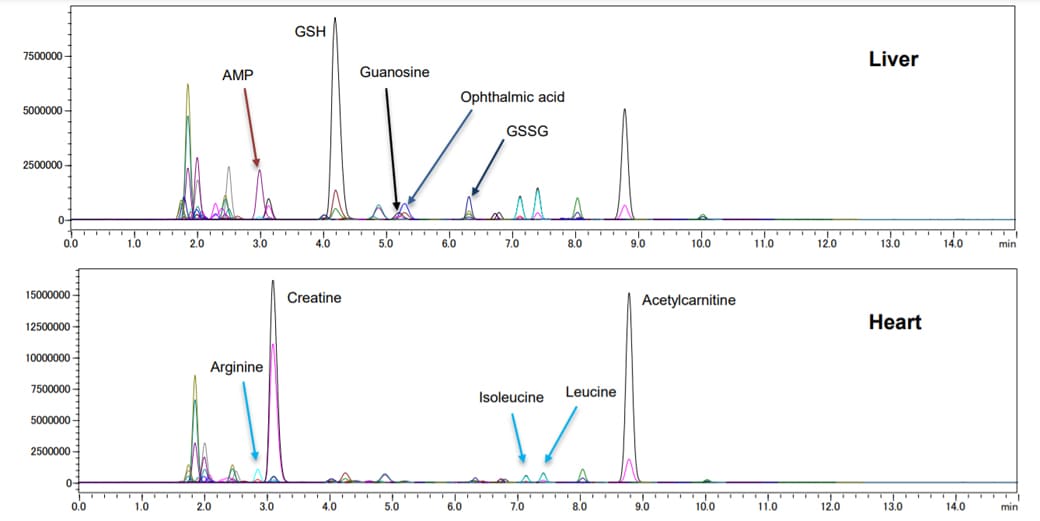
Fig. 1: MRM chromatograms for 97 primary metabolites, Upper: Liver, Lower: Heart
As an example of metabolite comparison on the metabolic pathway, Figure 2 illustrates the area ratio of organic acids in the TCA cycle of liver and heart tissue samples. Each ratio was calculated based on area of internal standard, 2-Morpholinoethanesulfonic acid in triplicate. The PFPP column was especially effective at separating amino and organic acids. The difference of relative ratio for organic acids on TCA cycle in two different tissues was confirmed.
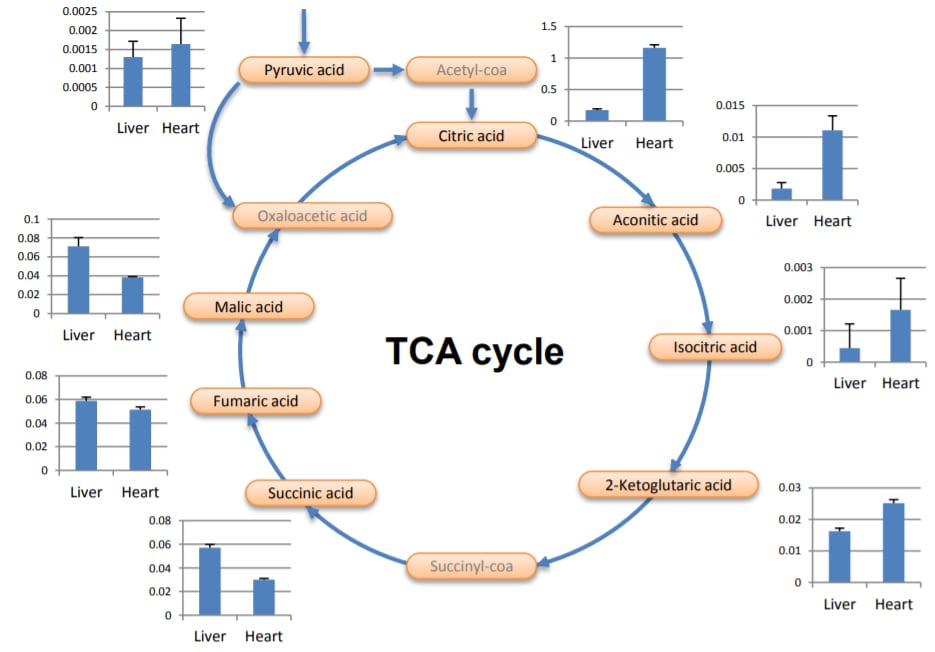
Fig. 2: Area comparison of organic acids in the TCA cycle between liver and heart tissue
Samples courtesy of Dr. Suematsu, Medical school, Keio University
LCMS-8040
The LCMS-8040 was designed to provide significantly higher sensitivity while maintaining the high speed offered by the LCMS-8030. Ultrafast MRM transition speeds, up to 555 MRMs per second (dwell times of 1 msec and pause times of 1 msec) are achieved.


